Should We Expect Super Bugs to Continu
The World Health Organisation has called antimicrobial resistance one of the greatest threats to human health. We explore how bacteria develop resistance to antibiotics, including how human behaviour is accelerating the process.
Table of contents
- A wonder drug is born
- Antibiotics resistance: one of the greatest threats to human health
- What are "superbugs"?
- Natural selection in action
- Gene pokemon
- Bacterial defence mechanisms against antibiotics
- Antibiotics: with great power comes great responsibility
- What can you do to combat antibiotic resistance?
- Article summary
A wonder drug is born

Many of us have heard the story of how Alexander Fleming inadvertently discovered Penicillin. It's a scientific discovery tale as iconic as Newton and the falling apple. For those who aren't familiar, here's a quick summary.
Fleming had been studying staphylococci bacteria, the microbe responsible for infections such as pneumonia. After returning from a holiday in 1928, he noticed a petri dish he had left out was full of mould.
Tellingly, the bacteria on the petri dish wouldn't grow near the mould, indicating that it was a powerful antimicrobial substance. As we now know, the mysterious substance was penicillin whose spores had floated through an open window.
Fleming published his findings and made preliminary efforts to engineer an antibiotic but abandoned the task for other study interests.
The Austrian scientist Howard Florey, along with his team at Oxford, rediscovered Flemings' work and extracted enough penicillin to trial on a human by 1941.
Their first patient was Albert Alexander, an off-duty police officer who cut his face on a rose bush and developed sepsis (a stark reminder of how vulnerable we were before antibiotics).
After being given penicillin, Alexander made a rapid and miraculous recovery, although Florey and his team ran out of penicillin after only four days and Alexander died shortly after.
With WW2 raging, the U.S. government began supporting the mass production of the drug, which became widely available a few years later.
Antibiotics resistance: one of the greatest threats to human health
Antibiotics revolutionised healthcare, rendering previously deadly bacterial infections easily treatable. But there is no such thing as a free lunch.
Bacteria are increasingly developing resistance to common antibiotics, with some species now non-responsive to the majority of antibiotics in our arsenal.
Parallel to this, large pharmaceutical companies are no longer pursuing antibiotic development as it is not economically viable. Instead, they are allocating funding to drugs taken for life, such as statins and antidepressants, which are far more profitable.
The halt in antibiotics research coupled with increasing bacterial resistance has created a perfect storm.
We now face a global antimicrobial resistance crisis, threatening to make routine medical treatments, such as c-section birth, transplantation and cancer therapy, highly dangerous.
Without antibiotics to safely facilitate these procedures, we could once again be at the mercy of bacterial infections.
The World Health Organisation has stated that AMR is one of the biggest threats to human health, estimating that it could claim 10 million lives annually by 2050 at the current rate.
What are "superbugs"?
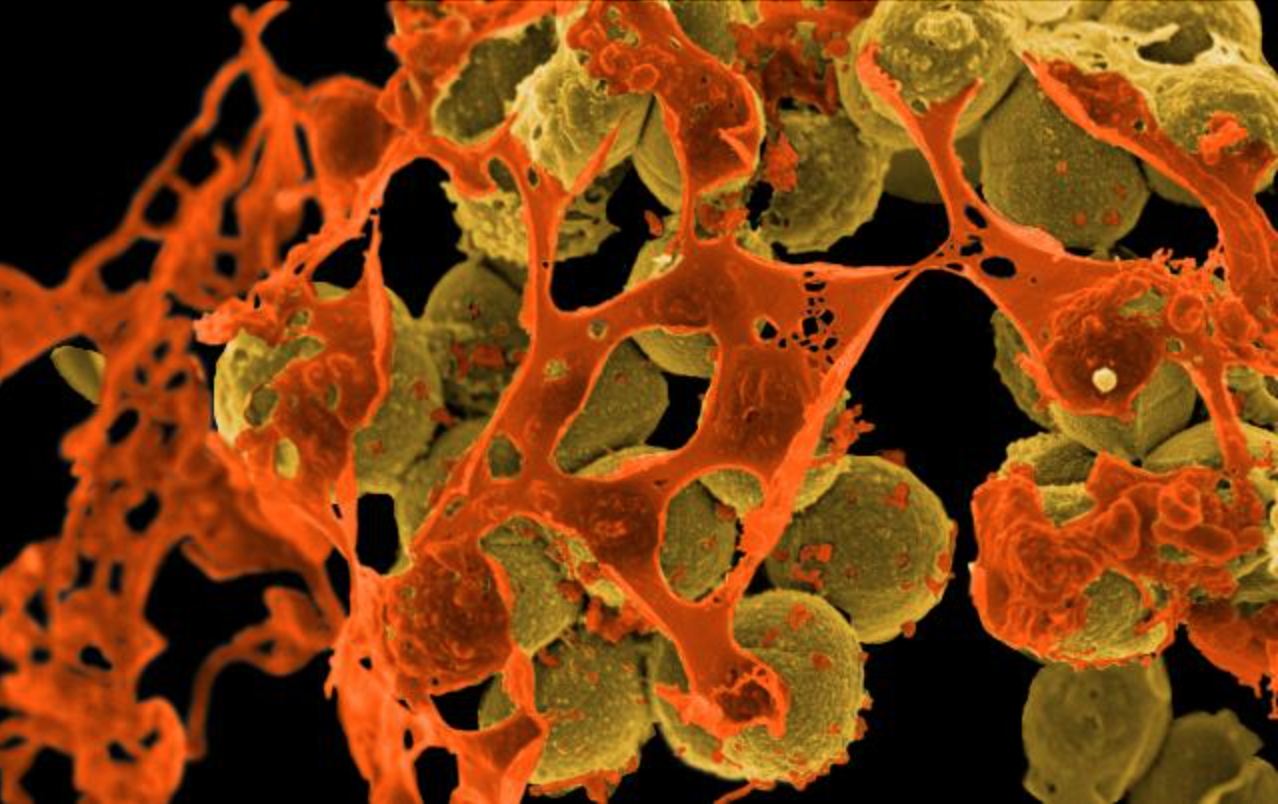
When bacteria develop antibiotic resistance, it is known as "antimicrobial resistance" or AMR.
Whist many bacteria are resistant to at least one antibiotic, germs are appearing which are resistant to multiple types of antimicrobial medicines. These are known as multi-drug resistant organisms, or colloquially as "superbugs".
Superbugs are growing more common, with several MDRA bacterial species known to science, including:
- *Methicillin-resistant Staphylococcus aureus (MRSA)
- Staphylococcus aureus (commonly known as Golden Staph)
- Multi-resistant Escherichia coli (commonly called E.coli)
- Clostridium difficile
- Neisseria gonorrhoeae
In his Nobel acceptance speech, Fleming presciently warned against this possibility, saying:
"…But I would like to sound one note of warning… The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily underdose himself and by exposing his microbes to non-lethal quantities of the drug make them resistant."
Bacteria began showing resistance within a decade of antibiotics introduction, but researchers simply outpaced this by developing new antibiotics faster than they could evolve.
Instead of solving the problem, however, it simply pushed it back for another generation.
Unfortunately, scientists have now harvested the "low hanging fruit" of antibiotics, meaning they have to be more creative in their hunt for new generation antimicrobials.
Not only is it costly and time-consuming to develop antibiotics, but it is not financially feasible for many pharmaceutical companies to do so.
There are two underlying causes behind antibiotic resistance and the emergence of superbugs, including natural selection and human behaviour. Let me explain:
Antibiotics-resistance: natural selection in action
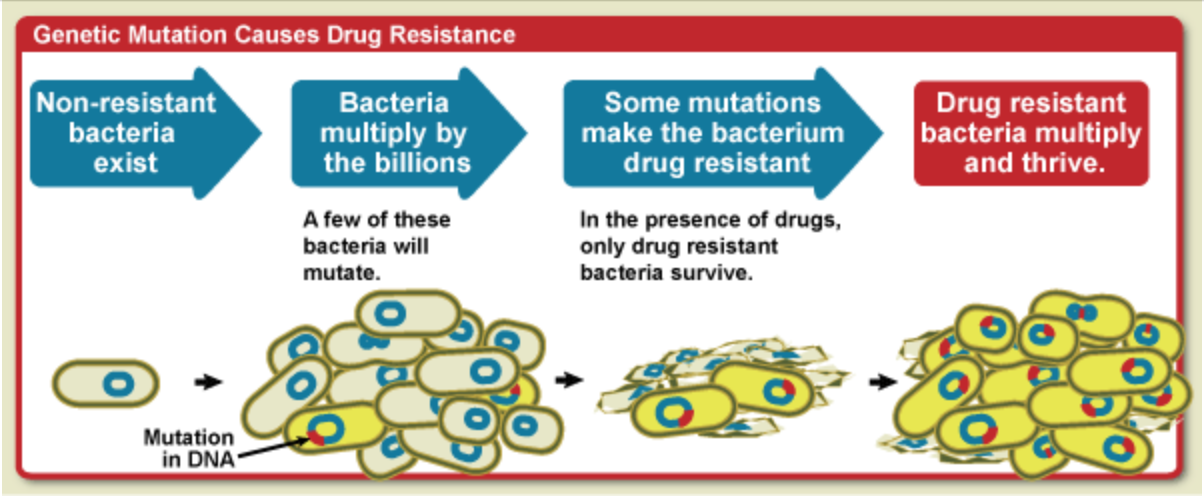
All living things undergo random genetic mutations, some of which are harmful, others harmless, and very occasionally, a few which are beneficial.
Bacteria, fungi and viruses reproduce far more quickly than other organisms (every 20 minutes for E-coli), meaning they experience more mutations in a shorter time frame. Moreover, germs that develop beneficial mutations can rapidly pass these genes to their offspring.
For a bacterium, acquiring resistance genes to antibiotics is like winning the lottery, granting them a massive survival advantage.
When you take an antibiotic, the offending germs should be killed or prevented from spreading, along with some unavoidable collateral damage.
But if a bacterium has a chance beneficial mutation, it will survive the antibiotics. In the vacuum left by the antibiotics, resistant bacterium can thrive and multiply, creating a new strain of germ immune to that antibiotic.
Each time you take antibiotics, there is a chance that certain bacteria will develop resistance to the drug. If this happens, it means the drug will be less effective or ineffective when taken in the future.
This is why it's essential to use antibiotics judiciously- if you take them when you do not need them, they might not work when you do.
Gene pokemon
Bacteria are the original life forms, meaning they have had plenty of time to develop clever survival techniques. One of their trade secrets is that they can share DNA with each other, like children swapping Pokemon cards in the playground.
Unfortunately for us, bacteria can exchange resistance genes with neighbouring bacteria, even those from different species. They do this via several clever mechanisms:
Firstly, bacteria can release genes when they die, which are picked up by passing microbes- like looting a body in a video game.
Secondly, bacteria can transfer bits of DNA through a mating process called conjugation, either directly or by forming "bridges" between one another.
Lastly, viruses called bacteriophages can transfer DNA between bacteria when they attack them.
Often shortened to phages, these are the sworn enemies of bacteria, acting like contract killers against specific species (more on these later).
Like children with their Pokemon cards, bacteria aren't limited to just one resistant gene- they can collect them all. The end result is superbugs resistant to multiple types of antibiotics.
Unless we act now, there is an urgent risk that a bacteria could emerge which is resistant to all the antibiotics at our disposal. If this happens, a scratch from a rose bush could once again prove fatal.
Bacterial defence mechanisms against antibiotics

There are roughly five types of defence mechanisms that bacteria (and other microbes) can acquire through mutations or gene transfer.
All of these confer some level of resistance to antibiotics, increasing the bacteriums chance of survival when you gulp down the drug.
The most resistant types of germ will possess more than one of these defence mechanisms, making them a fierce foe for antibiotics:
1.) Change the target area
Many antibiotics are designed to attack specific parts of the bacterium, like the cell wall. Germs can change the target so that the antibiotic no longer fits, like changing the locks on your house if someone gets their hands on a key.
2.) Learn to operate without the antibiotics target
Besides changing the area targetted by antibiotics, germs can develop new cell processes that bypass the targetted area. By doing so, the germs render antibiotic attacks non-critical to their functioning, just like the Black Knight in Monty Python yelling, "But a flesh wound."
3.) Inactivate or destroy antibiotics with proteins and enzymes
Germs can also produce enzymes and proteins which change or destroy antibiotics. Think of these as anti-aircraft guns that neutralise the threat before they attack.
4.) Develop extra pumps to remove antibiotics
Germs contain efflux pumps within their cell walls to expel threats. Some resistant bacteria possess extra pumps, allowing them to repel antibiotic attacks more efficiently.
5.) Change or decrease entry points
Lastly, germs can change or limit the entry points in their cell wall, fortifying their defences and confusing the attacker.
Antibiotics: with great power comes great responsibility
Antibiotic resistance is a natural response from bacteria to selective pressure, but the process has been accelerated by human behaviour.
More specifically, humans have used antibiotics inappropriately for decades, either prescribing them for issues they cannot treat or overusing them in general.
Historically, antibiotics have been routinely prescribed by doctors "just in case", or because patients expect to be given them.
A 2018 study by Public Health England estimated that GP's issue 20,000 unnecessary antibiotic prescriptions daily!
Worse still, antibiotics are often prescribed for viral infections like bronchitis and the common cold, neither of which antibiotics can actually treat.
The widespread use has given bacteria more opportunities to develop resistance and spread these genes to others.
The issue is further confounded by people not finishing their course of antibiotics and sharing prescriptions with others. As Fleming warned in his nobel acceptance speech, what doesn't kill bacteria can make them stronger!
Besides their overuse and misuse by humans, antibiotics are commonly given to livestock to prevent infection and fatten animals. Likewise, antibiotics are used in the agricultural industry to stop pests from damaging plants.
Not only does this give bacteria more opportunities to evolve resistance, but humans can pick up resistance bacteria when they ingest food products.
What can you do to combat antibiotic resistance?
We need to re-assess our relationship with antibiotics; otherwise, we risk undermining their effectiveness and ushering in a postbiotic era where resistant infections claim millions of lives.
Governments worldwide continue to allocate large amounts of resources to tackling antibiotic resistance, but we all have a part to play. Here are a few steps you can take to combat antibiotics resistance:
1.) Keep up to date with your vaccinations
By staying up to date with your vaccinations, you can avoid developing infections which require antibiotics in the first place, such as whooping cough and tetanus.
2.) Finish your pills
Even if your symptoms disappear, it is vital that you always finish a course of antibiotics as instructed by your doctor. Otherwise, you increase the risk of bacteria gaining resistance.
Have you ever watched a movie where the protagonist spares the villain, only for him to come back more dangerous than ever down the line? Not finishing a course of antibiotics is much the same.
3.) Don't share your prescriptions
Never share your prescriptions with others or take someone else's medication, as it increases the risk of bacteria gaining resistance.
4.) Don't take antibiotics for viral infections
Ask your doctor whether antibiotics are appropriate for your illness- if it's viral, then they cannot help.
Final thoughts
Antibiotics are a miracle drug, allowing bacterial infections that once killed millions to be treated easily.
What's more, antibiotics safely facilitate many standard operative procedures, from c-sections to organ transplants.
For too long we have abused these lifesaving drugs, giving bacteria the opportunity to develop resistance at a rate we cannot match.
To combat the growing threat of antibiotics resistance, we need to reassess our relationship with antibiotics, which means using them only when we absolutely need to- not for viruses, common colds or simply "just in case".
Whilst developing new antibiotics can keep us one step ahead of microbial resistance, it doesn't bring an end to the arms race with bacteria.
In light of this, researchers are looking for new, innovative ways to tackle bacterial infections and manage the growing threat of antibiotic resistance.
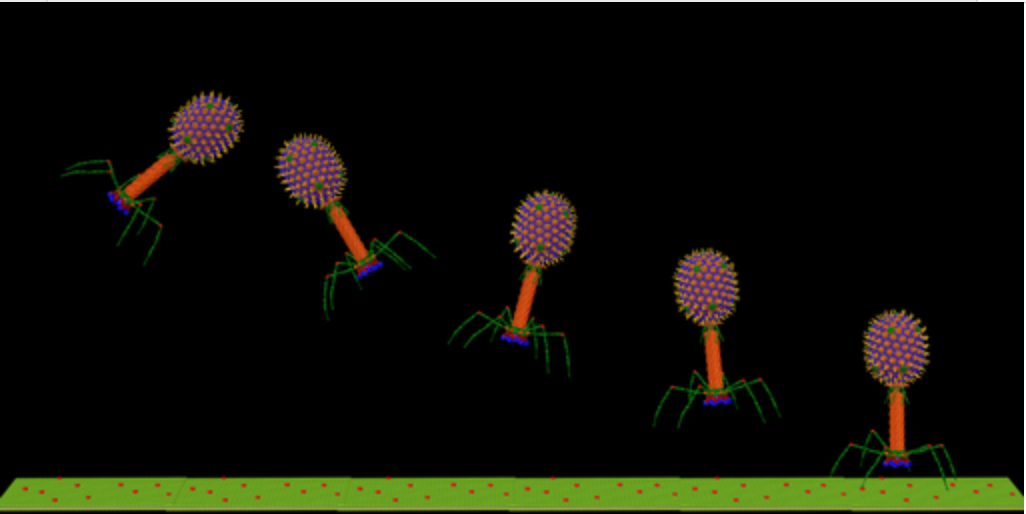
One potential solution to the problem is called phage therapy, whereby researchers use viruses programmed to kill specific bacterial species.
What's more, they cause far less collateral damage to beneficial bacteria than other antimicrobial agents; If broad-spectrum antibiotics are a nuclear bomb, these little guys are precision missiles.
Bacteria can and have developed defence mechanisms against bacteriophages, but phages can naturally evolve mechanisms to bypass these, unlike antibiotics.
Another potential solution to antibiotic resistance is something called quorum-targeting drugs. In short, bacteria don't mount an attack unless they have sufficient numbers.
Bacterial colonies communicate via quorum sensing, a system allowing them to rally an attack. Researchers are exploring the possibility of drugs which could keep bacteria permanently below the quorum threshold by disrupting quorum sensing.
Quorum-targeting drugs might be a way we can de-escalate the arms race with bacteria, keeping them pacified instead of waging all-out war and triggering resistance.
Article Summary
In a rush? We've got you covered. Here is a summary of the key points in this article:
- Antibiotics are used to treat bacterial infections by killing or preventing the spread of pathogens
- Antibiotics have been used so widely and for so long that numerous bacteria are gaining resistance, with new "superbugs" proving immune to the majority of antibiotics at our disposal
- Too few new antibiotics are being developed, meaning bacterial resistance is outpacing our ability to fight back
- Antibiotics resistance is a natural process of adaptation by bacteria in response to selective pressure, resulting from random mutations and gene transfer between bacteria
- Human behaviour is accelerating the issue; not only are antibiotics frequently misused for viral infections for which they aren't effective, but they are also extensively used in agriculture and livestock
- The World Health Organisation has called antimicrobial resistance one of the greatest threats to human health, warning that 10 million people could die annually from drug resistant infection by 2050 if nothing is done
- To prevent the dawn of a postbiotic age, researchers and governments are developing innovative new treatments to fight back. Two promising solutions including phage therapy and quorum-disrupting drugs
☝️DISCLAIMER☝This article is for informational purposes only. It is not intended to constitute or be a substitute for professional medical advice, diagnosis, or treatment.
Source: https://atlasbiomed.com/blog/superbugs-and-antibiotic-resistance/
0 Response to "Should We Expect Super Bugs to Continu"
Post a Comment